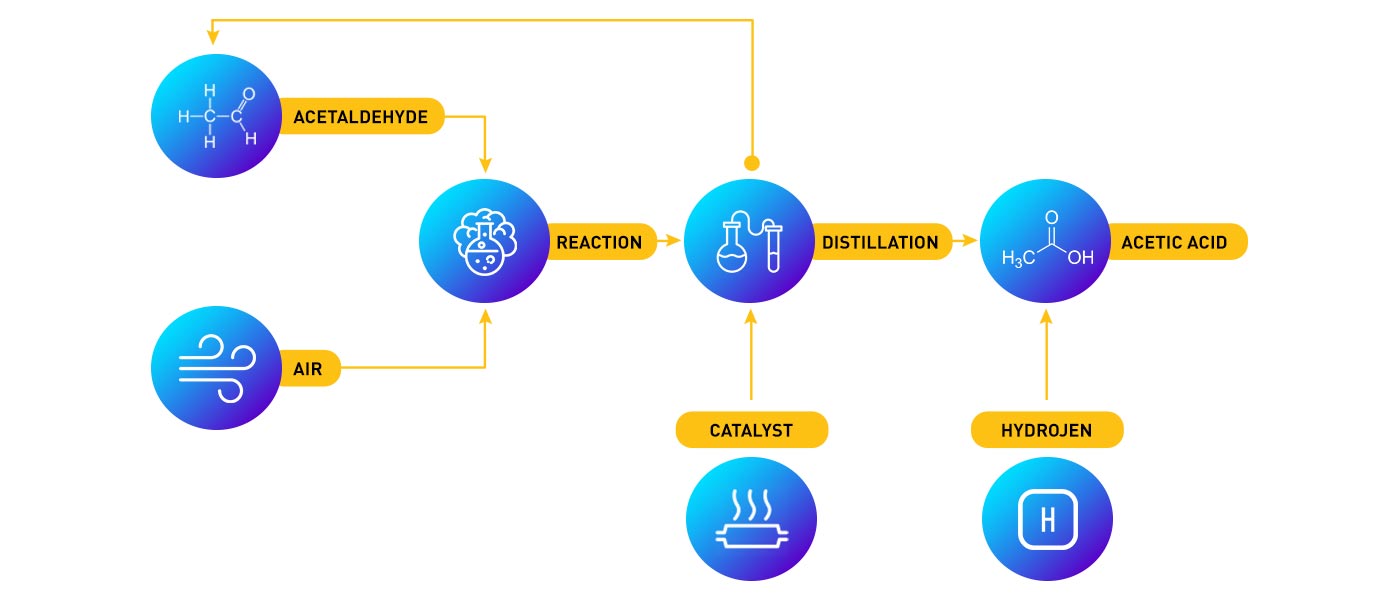
The ethanol is taken to the acetic acid plant and is first converted to acetaldehyde, thereafter to acetic acid. Acetic acid is produced by air oxidation of acetaldehyde in liquid phase using a dissolved catalyst. The reaction product containing unreacted acetaledyde, acetic acid and peracetic acid is heated and passed through another reactor where peracetic acid is reduced to acetic acid. Acetaldehyde is stripped away and recycled to the process. In a final distillation the water is removed leaving glacial acetic acid (99.5%) as the bottom product which is cooled and sent to storage.
Acetic acid is raw material for vinylacetate, acetic anhydride, cellulose acetate, acetic esters, glycol ether acetates, chloroacetic acid, PTA and Pharmaceuticals.