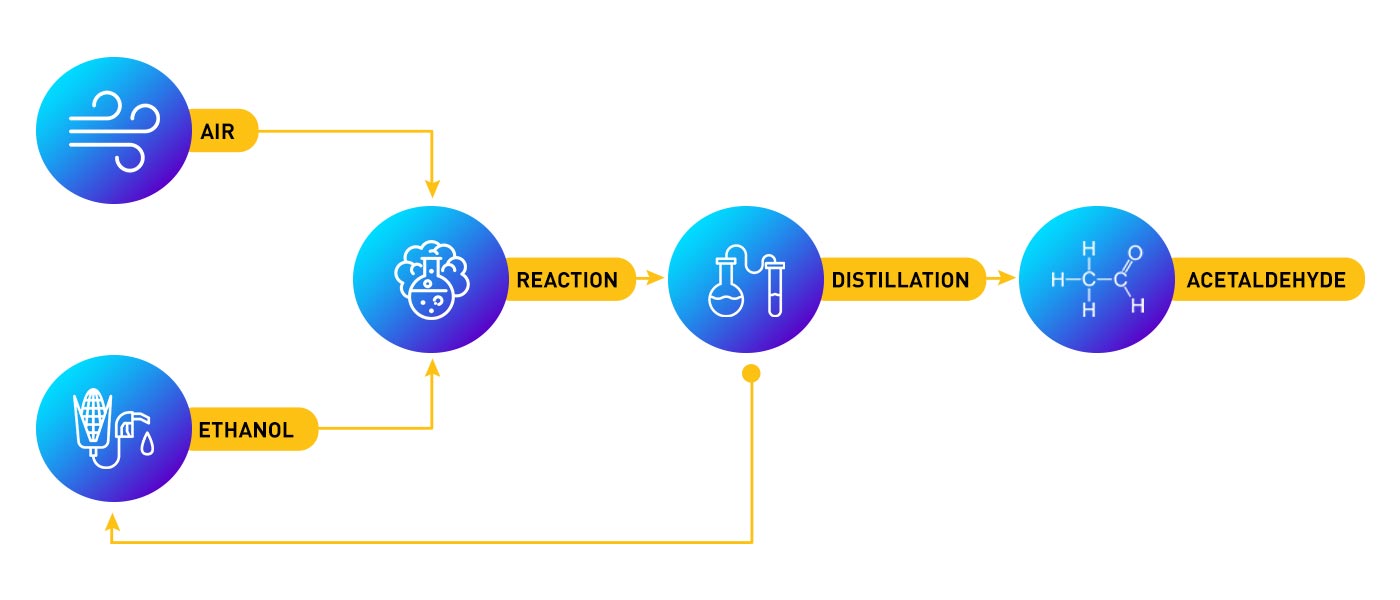
Acetaldehyde is produced by catalytic air oxidation in gas phase using a silver catalyst. The reaction is highly exothermic. The reaction mixture, containing mainly unconverted ethanol and acetaldehyde is absorbed in an ethanol water mixture which is distilled to give acetaldehyde. Ethanol is recovered from the bottom stream and recycled to the process.
Acetaldehyde can be sold as a product or further processed to acetic acid. Acetic acid is produced by air oxidation of acetaldehyde in liquid phase using a dissolved catalyst. The reaction product containing unreacted acetaldehyde, acetic acid and peracetic acid is heated and passed through another reactor where peracetic acid is reduced to acetic acid. Acetaldehyde is stripped away and recycled to the process. In a final distillation the water is removed leaving glacial acetic acid (99.5%) as the bottom product which is cooled and sent to storage.