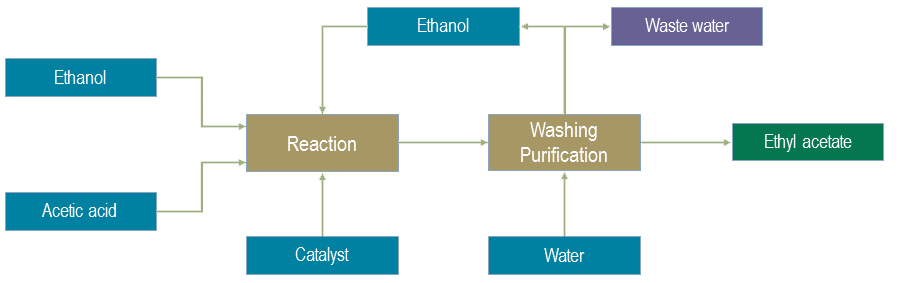
Ethyl acetate is produced by esterification of acetic acid with ethanol using sulfuric acid as catalyst. Ethanol and acetic acid are continuously fed to the steam heated reactor. The ethyl acetate formed is removed from the top of the reactor column together with some ethanol and water. The oily phase is separated and washed and then sent to the ester column where the product is removed from the bottom. Water phases from decanters are sent to the recovery column where ethanol is recovered and sent back to the reactor. The bottom water phase is sent out as effluent.
Ethyl Acetate, CAS No. 141-78-6 and molecular formula C4H8O2, is a colorless liquid and has a characteristic sweet smell. It is used as solvent for paints, extraction agent, raw material for pharmaceuticals, cosmetics and polishes.